КОНВЕРСИЯ МЕТАНОЛА В -ОЛЕФИНЫ В ПРИСУТСТВИИ МОДИФИЦИРОВАННЫХ ЦЕРИЕМ ЦЕОЛИТНЫХ КАТАЛИЗАТОРОВ ТИПА ZSM-5
КОНВЕРСИЯ МЕТАНОЛА В -ОЛЕФИНЫ В ПРИСУТСТВИИ МОДИФИЦИРОВАННЫХ ЦЕРИЕМ ЦЕОЛИТНЫХ КАТАЛИЗАТОРОВ ТИПА ZSM-5
Аннотация
Для повышения селективности по отношению к легким олефинам, в частности пропилену, были синтезированы каталитические составы на основе цеолита ZSM-5 и церия с использованием метода твердофазной модификации, а их каталитические характеристики были исследованы в процессе конверсии метанола в олефины. Конверсию метанола в углеводороды проводили в аппарате проточного типа, используя стационарный слоистый катализатор, в температурном диапазоне 250–550°C, при объемной скорости подачи метанола 2,0 при атмосферном давлении и в присутствии газообразного азота.
Было продемонстрировано, что после модификации наночастицы оксида церия дисперсно распределяются на внешней поверхности и в порах цеолита, взаимодействуя с его кристаллической решеткой, что приводит к уменьшению плотности сильных кислотных центров. Плотность центров сильных кислот в цеолите зависит от концентрации церия. Увеличение концентрации церия в цеолите до 5,0 масс. % приводит к снижению селективности по отношению к этилену. Примечательно, что высокая селективность по пропилену (39,7%) наблюдается в 4%-ном Ce-HZSM-5 при 550 °С, в то время как значительная селективность по бутилену (23,1–23,7%) отмечается в 5%-ном Ce-HZSM-5 при температурах 400–450 °С. Кроме того, значительная селективность по -олефинам (72,4%) достигается при использовании катализатора 4% Ce-HZSM-5 при 500°C. Наблюдаемое увеличение селективности по пропилену и бутилену можно объяснить уменьшением плотности центров сильных кислот в результате процесса модификации.
1. Introduction
C2_C4 olefinic hydrocarbons are essential precursors in the petrochemical industry, primarily produced through the pyrolytic breakdown of oil fractions, thermal cracking, and the dehydrogenation of light alkanes using traditional methods. Recently, significant attention has been focused on the recovery of valuable olefins, aromatic compounds, and isoparaffins from alternative regenerated feedstocks, particularly in the presence of high-silica ZSM-5 type zeolite catalysts , , , . The production of light C2_C4 olefins and hydrocarbons within the gasoline range from natural gas, which serves as a feasible alternative to petroleum, through synthetic gas and methanol derived from biomass, represents one of the most effective strategies. Currently, Mobil Oil Corporation has initiated the production of C2_C4 olefins utilizing catalysts based on SAPO-34 molecular sieves in the Methanol-to-Olefins (MTO) process. SAPO-34 has demonstrated efficacy as a catalyst for the selective conversion of methanol to ethylene, achieving a 100% conversion rate under mild operational conditions. However, achieving high yields of propylene remains a significant challenge , , , . Among the various molecular sieves, the ZSM-5 type zeolite employed in the MTO reaction is characterized by its network of intersecting salt (0.51x0.53 nm) and sinusoidal (0.53x0.55 nm) nanoscale channels, along with a high specific surface area and remarkable resistance to acidic conditions and deactivation, making it particularly promising. Numerous studies have investigated the effects of different metallic species on the catalytic properties of ZSM-5 zeolite catalysts, aiming to enhance selectivity through variations in charge distributions. ZSM-5 zeolite catalysts modified with metals such as Ca, Cr, Cu, Li, Mg, Ni, and Cu show significant selectivity towards light olefins. Among the modified ZSM-5 zeolite variants with Mn, Cr, Fe, Ni, and Ag, the Mn/HZS variant has been noted for its performance , , , .
ZSM-5 zeolite can undergo modification using phosphorus compounds such as metaphosphoric acid, ammonium dihydrogen phosphate, and trimethyl phosphite to enhance its selectivity for light olefins. A notable yield of 47.01% propylene was achieved from methanol conversion when utilizing a ZSM-5 zeolite catalyst that had been modified with 90% phosphorus , , . This zeolite effectively transforms methanol into light C2-C4 olefins and p-xylene with significant selectivity following solid-phase modification with Neodium oxide .
Furthermore, ZSM-5 zeolite catalysts that have been modified with non-transition elements (NTE), including La, Ce, Pr, Nd, Sn, and En, demonstrated considerable activity for producing C2-C4 olefins during butane cracking. In light of these findings, this study investigates the influence of cerium concentration on the catalytic performance of ZSM-5 zeolite in the methanol conversion process, particularly focusing on the production of light C2-C4 olefins, with an emphasis on propylene , .
2. Experimental part
Commercial zeolite ZSM-5 (SiO2/Al2O3=40; Na2O<0,05 wt.q) was used to prepare the catalysts. The catalytic compositions were prepared by solid-phase modification of cerium carbonate with HZSM-5 zeolite in a ball vibrating mill for 2 hours followed by calcination at 300° and 500°C for 4 hours respectively. All samples were pressed, and pulverized, then a 1,0–1,5 mm diameter fraction was selected for testing. The content of cerium catalytic composition was 1,0–5.0 wt.q RFA of the syntesited catalysts was carried out using a RIGAKU “MINIFLEX” X-ray diffractometer with CuKt radiation according to the method described in
.The catalytic experiments were carried out in a flow tubular quarts reactor (length 10 cm, inner diameter 1,0 cm) with a stationary bed loaded with 2.0 g of catalyst, a volumetric feed rate of methanol in the temperature range of 300°–550°C.
2.0 h-1 in the presence of nitrogen (CH3OH/ N2=0.33 mol). The reaction products were analyzed on an Agilent GC782A gas chromatograph according to the procedure described in
.3. Result and discussion
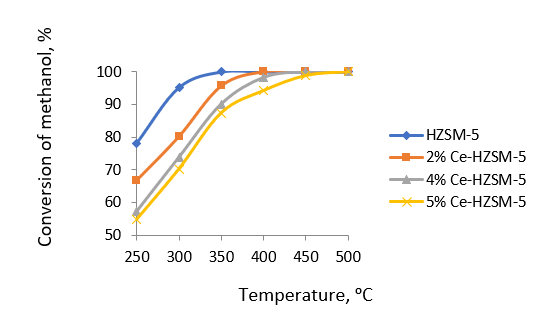
Figure 1 - Temperature dependence of methanol conversion on HZSM-5 and modified samples
It is evident that an increase in cerium concentration within HZSM-5 zeolite to 5.0 wt% corresponds with a decline in the concentration of robust acid sites, decreasing from 235 μmol/g to 114 μmol/g.
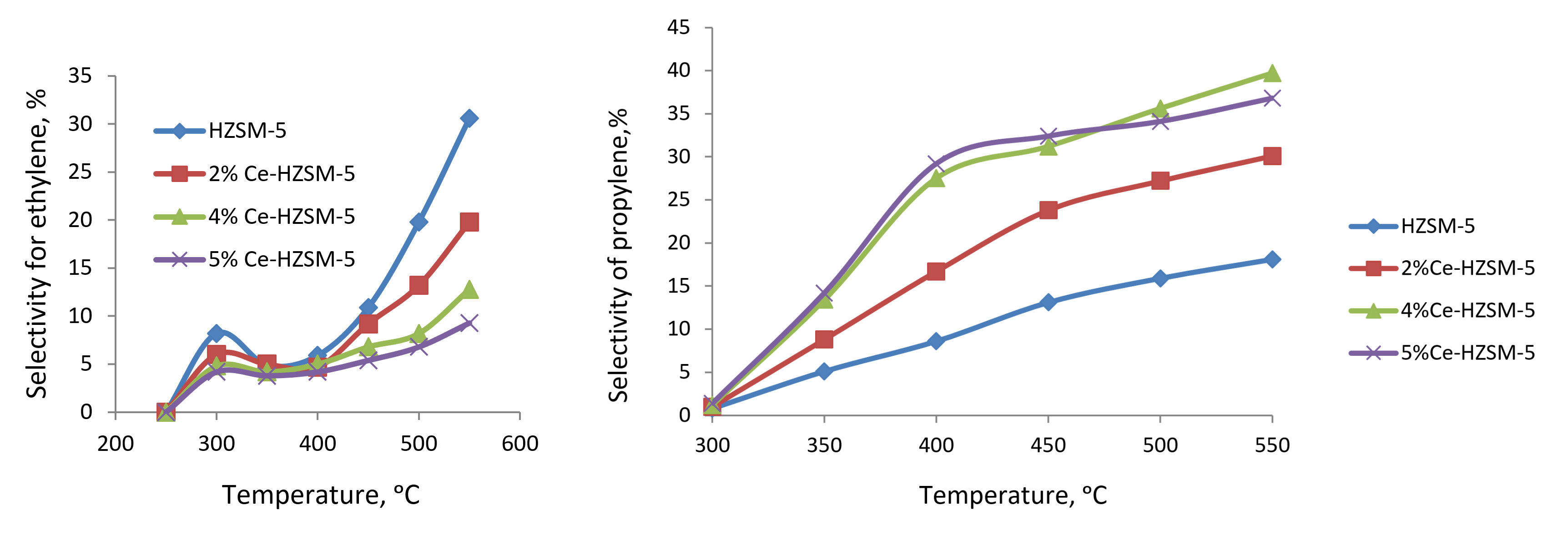
Figure 2 - Temperature dependence of selectivity for ethylene and propylene on HZSM-5 and modified samples
Table 1 - Data table
Type of catalyst | Weak acid sites, µmol/g (100-300°C) | Strong acid sites, µmol/g (300-600°C) | Total concentration of acid sites µmol/g |
HZSM-5 | 394 | 235 | 629 |
2% B-HZSM-5 | 316 | 182 | 498 |
4% B-HZSM-5 | 267 | 138 | 405 |
5% B-HZSM-5 | 246 | 114 | 350 |
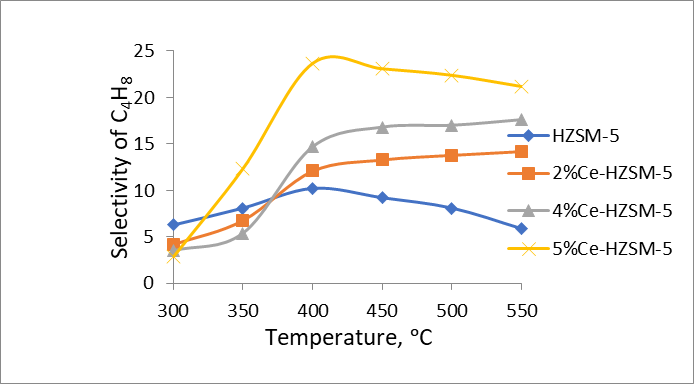
Figure 3 - Temperature dependence of selectivity for butylene on Ce/HZSM-5 and modified samples
Analogously, an increase in the cerium content within HZSM-5 zeolite correlates with a higher selectivity for butylene. A heightened selectivity (30.6%) for ethylene is observed. The specimen modified with 5.0 wt % cerium demonstrates an elevated selectivity for butylene.
4. Conclusion
1. Catalytic systems were obtained by solid-phase modification of zeolite HZSM-5 with cerium oxide in order to study the effect of cerium oxide concentration on the selectivity of C2-C4 olefins formation from methanol.
2. It was found that the selectivity for C2-C4 olefins in methanol conversion in the presence of CeO2/HZSM-5 is determined by the concentration of cerium oxide in the catalyst and the density of acid centres of the zeolite.
3. The optimal compositions of catalysts in the presence of which high yields of propylene and C2-C4 olefins are achieved were revealed. The catalyst containing 4% CeO2 provides maximum selectivity (39.7%) for propylene at 500°C, and on the catalyst of 5%CeO2/HZSM-5 composition at 450°C maximum selectivity (72.4%) for C2-C4 olefins is achieved.
