Polymerization of vinyl acetate in the presence of polylactide-poly(ethylene glycol) block-copolymers
Polymerization of vinyl acetate in the presence of polylactide-poly(ethylene glycol) block-copolymers
Abstract
This paper presents data on the polymerization of vinyl acetate in emulsions stabilized with biodegradable water-insoluble linear polylactide-poly(ethylene glycol) block-copolymers. Since PEG is a readily soluble, highly polar polymer capable of forming a random coil, which can effectively sterically protect the surface of various nanoparticles and microparticles, block copolymers with various hydrophobic blocks can be obtained using PEG of different functionality and molecular weights. Diblock-copolymers were prepared from poly(ethylene glycol) methyl ether (MPEG, Mn = 2 000 Da) and L-lactide. The colloid-chemical properties of the copolymers have been studied. The block-copolymer was then used as the surfactant for the emulsion polymerization of vinyl acetate in the presence of potassium persulfate as an initiator. The effects of a new polymeric emulsifier on the physicochemical properties of obtained latexes were investigated.
1. Introduction
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)) has a number of valuable specific properties and is widely used in various fields – from household products to materials for medical and biological purposes. The most important qualities of PVA are its universal adhesive and binding properties, high strength of fibers and film materials made with its use .
2. Research methods and principles
The main method for producing PVA is emulsion polymerization. Among emulsion monomers, vinyl acetate (VA) stands out for its good solubility in water in contrast to vinylbenzene, and this what determines the patterns of PVA synthesis , , . The considerable reactivity of the VA radical results in heightened susceptibility to the existence of impurities, it is active in chain transfer reactions, and usually VA polymerization proceeds with an induction period , .
In radical polymerization of vinyl acetate, anionic and nonionic surfactants are most often used , . A number of studies have demonstrated the existence of nonionic high-molecular surfactants, for instance, pluronics (triblock-copolymers of a polyoxypropylene and two hydrophilic chains of polyoxyethylene). This can lead to promising stability for PVA particles , , . According to multiple studies, the importance of environmental protection was highlighted by examining various factors, high-molecular surfactants that can decompose under natural conditions to innocuous low-molecular products are promising. These are amphiphilic block-copolymers based on lactic acid , , , .
Thus, in this work, we synthesized several biodegradable linear amphiphilic block-copolymers of L-lactide and ethylene glycol. The colloid-chemical properties of those synthesized compounds were evaluated, and the heterophase polymerization of vinyl acetate in their presence were studied.
3. Main results
3.1. Synthesis of the linear diblock-copolymers
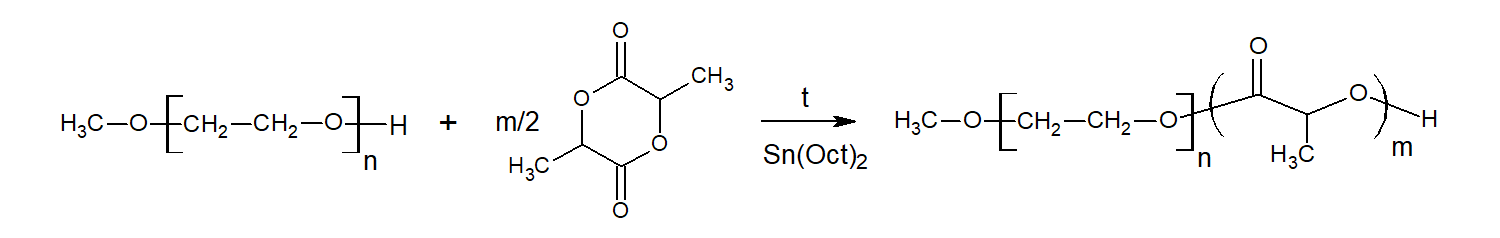
Figure 1 - Reactions pathways in the synthesis of PLLA-MPEG block-copolymers
Table 1 - Characteristics of polylactide-poly(ethylene glycol) block-copolymers
Note: aDetermined by 1H-NMR. bDetermined by GPC (eluent: THF at 40 °C)
The colloidal chemical characteristics of PLLA-MPEG block-copolymers were investigated. The interfacial tension and surface tension of the solutions were determined at room temperature by using a KRŰSS K9 surface tension tensiometer. It has been discovered that all copolymers decrease the interfacial tension to levels below 20 mN/m, aligning with the information found in the literature regarding copolymers containing polylactide , , .
3.4. The use of synthesized copolymers in the polymerization of vinyl acetate as surfactants
Linear and hyperbranched poly(ethylene oxide)-containing copolymers with high biocompatibility, obtained on the basis of aliphatic copolyesters, whose hydrophilic blocks are formed by polyethers, and hydrophobic blocks are formed by polymers of hydroxyacids (glycolic, lactic, hydroxybutyric, etc.), can be ranged among such biodegradable surfactants. It is known that the hydrolysis of such amphiphilic macromolecules leads to decomposition into environmentally harmless natural hydroxyacids and biocompatible oligomers , , , .
The use of water-insoluble surfactants in the heterophase polymerization of vinyl monomers is seen as a promising approach to producing polymer suspensions with a narrow particle size distribution (PSD). Biodegradable polyesters have been demonstrated to be exceptional stabilizers of polymer microspheres and hold a distinctive position among water-insoluble surfactants , .
The PLLA60-MPEG45 block copolymers were employed as a surfactant in the emulsion polymerization of vinyl acetate with potassium persulfate (PPS) as the initiator. Vinyl acetate (Fluka) with a basic substance content of ≥99 % was used as a monomer, and PPS (Sigma-Aldrich) with a basic substance content of 99.9% was used as an initiator. The vinyl acetate polymerization took place at a temperature 60±0.5 ⁰C using a monomer-to-water volume ratio of 1:9. The initiator concentration was 1 wt % based on VA, while the surfactant concentration was 1.0 wt % per monomer.
3.5. Evaluation of the polymerization process using dilatometry method
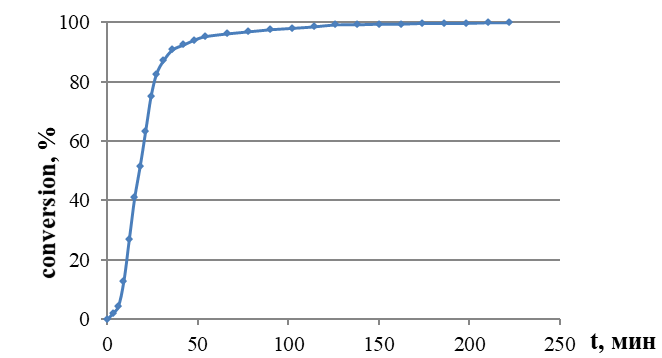
Figure 2 - Conversion – time curves obtained for vinyl acetate (VA) polymerization at 60 °C in the presence of PLLA60-MPEG45
Note: volume ratio of VA : water = 1 : 9, [surfactant] = 1.0 wt %, [PPS] = 1.0 wt %
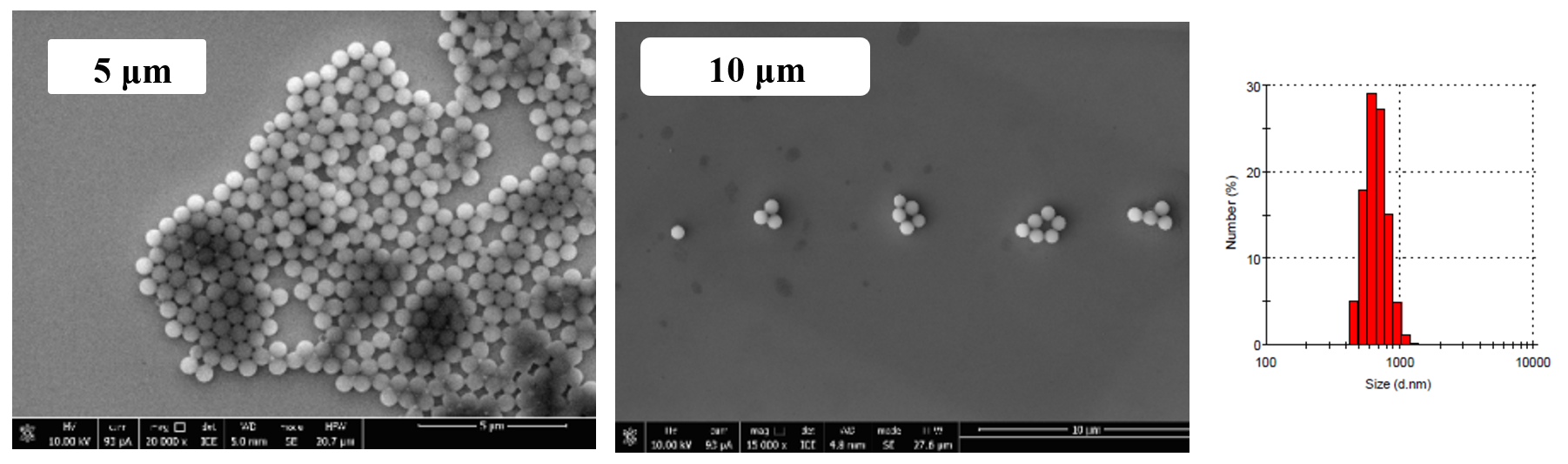
Figure 3 - Photomicrographs and histogram of particle size distribution of polyvinyl acetate suspension
4. Conclusion
Thus, the synthesized biodegradable linear amphiphilic block-copolymers of L-lactide and ethylene glycol were comprehensively investigated. The high surface activity of polylactide-poly(ethylene glycol) block-copolymers and their capacity to generate thick interfacial adsorption layers on the polymer particle surfaces provide a rationale for the system's stability during polymerization.
